Standards for electrolyte density in a battery
To understand what density should be in a battery, you need to familiarize yourself with the standards that determine the permissible acid content in the working solution. It is assumed that this indicator is measured in grams per cubic centimeter. Practice has shown that any battery works normally as long as the density of the electrolyte in its banks remains at the level of nominal values from 1.23 to 1.30.
Important! The large spread of this indicator indicates that it can change over time while remaining within acceptable limits.
Thus, for the summer and winter seasons, for example, the standardized indicators of electrolyte density differ significantly. To find out whether the measured value corresponds to the norm, you need to do the following:
— compare the normalized density of the electrolyte in the battery in winter, for example, with the outside temperature;
— take into account the recommendations of battery manufacturers, indicating the dependence of its performance on climatic conditions;
— when determining the exact value of the indicator, be guided by a special table attached to the battery in the form of a sticker on the case (sometimes it is given in the passport or in the user manual).
From the tables it can be seen that a maximum density of 1.30 corresponds to a temperature below minus 30 degrees, and a value of 1.28 is tied to the range from -10 to -30. As it rises, this parameter drops to 1.26, and with positive values it should be 1.23 (this is the minimum indicator).
Please note: All of the above data is valid only for well-charged batteries.
As a rule, they are not adjusted for temperature changes over a short period of time (during the day, for example). A rare exception to this rule are the harsh climatic conditions of Siberia and the Arctic, as well as frosty winters in central Russia.
How to check the battery at home
Operation of the machine must be accompanied by periodic checking of the condition of the battery. As mentioned earlier, an increase in the concentration of sulfuric acid in banks negatively affects the performance of the battery. Car service technicians recommend checking the technical condition of the battery every 15-20 thousand kilometers. Diagnostics can be carried out as follows:
- Inspect the battery case for mechanical damage and leaks.
- Visually assess the electrolytic fluid level. It should rise 1.3 cm above the edge of the jars.
- Measure the electrolyte density using a test instrument.
Sometimes the charge may decrease because the alternator drive belt is loose. Therefore, car owners are recommended to regularly check its tension themselves or at a service center.
Measurements using a hydrometer
As mentioned earlier, measuring the density of the electrolytic substance in the battery is carried out with a hydrometer. Before measuring procedures, it is necessary to assess the level of special fluid in each battery plate and correct it with purified water. After this, the battery needs to be fully charged and the test started, guided by the following algorithm:
- Place the battery on a flat surface.
- Remove the plug on the filler cap.
- Immerse the hydrometer in the electrolytic liquid and draw it into the device using the rubber tip.
- Add enough reagent to move the float.
- Measure the density with a hydrometer, guided by the readings on a special scale.
- Record the result obtained and repeat the procedure on the remaining plates.
- Compare the obtained data with standard values.
It is noteworthy that the values in all banks must be the same. If the measurement shows that the density in individual plates is reduced, it is necessary to check the battery for damage or short circuits between the banks. Low density in all containers may indicate that the battery is severely discharged or worn out. Operating a car with such a battery will be difficult.
Is it possible to take measurements without a hydrometer?
It will not be possible to carry out tests with electrolytic liquid without a special measuring device. At the same time, you can make a hydrometer with your own hands by preparing a float-weight in advance. To create a device yourself, you must perform the following steps:
- Prepare a hollow plastic tube, such as a drinking straw.
- Place the pre-prepared float in purified water, noting the liquid level on it. This mark will correspond to 1 g/ml.
- Place the float in new electrolytic fluid with a precise specific gravity, such as 1.4 g/cm3.
- Make a mark where the electrolyte comes into contact with the float, and then mark the distance between the two available values.
To select the electrolytic fluid, you can use a rubber bulb. As an alternative, a regular glass is used where the float is placed.
Carrying out measurements on a maintenance-free battery
The problem with measuring electrolyte density in maintenance-free batteries is the lack of unscrewable plugs. However, when making batteries, manufacturers use holes that are later sealed with disposable plugs. If necessary, they can be dismantled or drilled, turning a maintenance-free battery into a serviceable one.
It is noteworthy that after servicing the battery, the holes must be securely sealed. This is done using plastic, since it easily melts and sticks to any materials. Therefore, even if it is not possible to completely remove the plugs, they can be drilled and sealed with plastic after servicing.
Video of electrolytic fluid measurements
To understand how the electrolyte density in a battery is measured, just watch the video below:
This video discusses the process of taking measurements using a hydrometer. As mentioned earlier, a similar procedure can be carried out with a homemade measuring tool.
Methods for increasing the electrolyte density in a serviced battery
Car enthusiasts know that rechargeable batteries are divided into serviceable and maintenance-free samples, and in a second class battery it is impossible to control and change the electrolyte density. Therefore, questions about its increase concern only serviced samples that provide free access to each jar.
In a situation where obvious damage is found on the battery (cracks or chips, for example), you should not even try to restore its functionality. In this case, it is wiser to purchase a new battery, which will allow you not to waste your personal time.
If no defects are found on the body, and the density has decreased only slightly, the following ways to increase it are possible:
— use a special electronic device (CHD) that can be used to charge the battery;
— if recharging does not help and the car continues to start with difficulty, you will have to use the method of adding electrolyte (followed by a full charge);
— in a situation where both methods did not lead to the required result (the density still remains low), a radical approach will be required, consisting of a complete replacement of the electrolyte.
Once the solution is updated, you can proceed to restoring the battery using charging current. Let's consider each of the options in more detail.
Recharging
This approach to restoring the battery involves charging it with a weak current to a state that complies with current standards (the voltage at the terminals should be about 13-14 Volts).
Important! Recharging should take at least 12 hours.
If necessary, you will have to repeat this procedure, charging the battery at a voltage of 14.6 Volts, but with a higher current (up to 2 Amperes). After it has settled, a control measurement of the operating parameter is carried out. Periodic recharging will increase the density of the solution to normal values.
Adding Electrolyte
To carry out this procedure you will need:
- Density meter - hydrometer.
- Rubber bulb for selecting and adding solution.
- Special measuring container.
- Reservoir for old solution.
In addition, it is necessary to prepare a supply of correction electrolyte and distilled water. They will allow you to bring the solution to the desired condition.
The procedure for increasing density is as follows:
- First, from a jar in which the density is below the standard value, the solution is sucked out using a bulb (the more, the better).
- Then the volume of the selected liquid is measured, for which it is poured into a measuring container.
- After this, fresh electrolyte is added to the emptied jar in a volume equal to half of the withdrawn one. The battery itself is shaken from side to side to mix the resulting mixture.
- Next, you should measure the density of the composition and, if necessary, add electrolyte in the volume of a quarter of the previously pumped out solution.
- This procedure is repeated until the density becomes normal, after which the lack of liquid is replenished with a portion of distilled water.
Similar actions are performed with all other banks.
After this, they are left to settle for a couple of hours, which ensures that the solution in the tanks is leveled to a homogeneous state. And only after that its density is measured again.
Please note: In a situation where the densities in different banks are even slightly different, they will need to be adjusted towards equalization.
To do this, it is necessary to continue charging with a current whose rating is two or even three times less than optimal.
Electrolyte renewal
It will no longer be possible to increase the density of the electrolyte when its indicator has reached a critical level of 1.18 units with the old solution. In this situation, you will have to completely update it. To restore the battery's functionality, you will need to do the following:
- pump out the liquid from all the cans using a rubber bulb (as far as possible);
— return all the plugs to their place and install the battery so as to provide access to the bottom without turning it over;
— drill a small hole in the bottom of each jar, after placing a suitable container for collecting waste;
- unscrew the plugs again and rinse the insides with distilled water;
- solder the hole with an acid-resistant compound;
— fill the jars with new solution and charge the battery (as described above).
Additional information: After filling, the electrolyte level should overlap the battery plates by approximately 10-15mm (the quality of its charging will depend on this).
The procedures performed in accordance with these instructions will extend the life of the battery for several years.
How to measure
First of all, you need to fully charge the battery. The further procedure should be carried out at a temperature of +22..+28 degrees, so the battery must be kept in a room with such conditions for at least an hour. In practice, ideal conditions are not always present, so in many cases you will have to make a correction for temperature - as it increases, the density decreases (with a constant concentration), and as it decreases, it increases. The amendment can be taken from the table.
| Liquid reagent temperature, degrees C | Correction to instrument readings, g/cc. |
| -25..-11 | +0,03 |
| -10..+4 | +0,02 |
| +5..+19 | +0,01 |
| +20..+30 | 0 |
| +31..+45 | -0,01 |
| +45 and above | -0,02 |
So, if, when measuring at a temperature of minus 15 degrees, the hydrometer showed 1.25 g/cc, this means that 0.03 must be added to the instrument readings; under normal conditions, the density will be 1.28 g/cc.
To measure density, a special device is used - a hydrometer. Any one that allows you to measure a density of more than 1 g/cc will do, but it is better to use a special one for automotive electrolyte. Its measurement limits are usually 1.1..1.3 g/cc (with a scale acceptable for accurate reading), which covers almost all cases that can be encountered in practice. A household alcohol meter will not work - most of these devices are designed to measure density below 1 g/cc.
Modern hydrometers are combined with a sampling bulb (this device is also called a densimeter). If it is not there, you will need an additional rubber bulb and a glass made of chemically inert material. First you need to draw a sufficient amount of electrolyte into their jars.
Collecting electrolyte from a battery can with a densimeter.
If the device is combined, you can immediately read the measured value on the scale. If it’s normal, pour the electrolyte from the pear into a glass and measure it. Readings are taken at the mark corresponding to the liquid level. Then you need to return the electrolyte back to the battery jar.
The density of the liquid reagent must be measured in each battery cell separately.
Reading hydrometer readings.
Maintenance-free battery design
Products of this type do not have holes for adding liquids and solutions, since their body is completely sealed. A modern maintenance-free battery consists of the following parts:
- Working plates.
- Separator layers.
- Contact pins.
- Sealed housing.
- Lid.
The galvanic cells placed in it are chemical sources of current. They are placed in a monolithic case and separated by partitions (photo on the right).
Important! They are reversible energy sources that can be charged and discharged a large number of times.
The design features of such batteries allow them to be used for quite a long time.
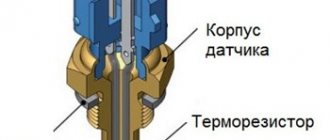
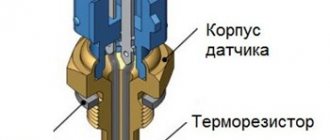
What is a hydrometer
A device for measuring the concentration of various solutions is called a hydrometer. The operation of the density meter is implemented on the basis of a well-known physical law.
Archimedes' principle states that if a body is immersed in a liquid, a hydrostatic or lifting force will begin to act on it. In modulus it will be equal to the weight of the liquid that the body displaced, but opposite in direction.
For practical use, the hydrometer scale is graduated to the concentration of the dissolved substance. Car enthusiasts will find a useful device for checking the density of electrolyte and antifreeze.
How to measure density
Before checking the battery density, you should be well prepared for this procedure. To carry it out successfully, you will need a proprietary hydrometer in the form of a transparent glass flask with a built-in scale. When immersed in an electrolyte, it will be pushed out of the liquid the more strongly, the greater the density of the controlled solution.
Important! This indicator should be checked only when at least 6 hours have passed since the battery was charged.
The procedure for checking it is as follows:
- First you need to unscrew all the caps of individual cans.
- After this, the measuring device is taken and lowered strictly vertically into a separate battery cell.
- When the hydrometer calms down a little and stops moving vertically, you should read the reading on its scale.
The results of self-conducted measurements can be considered satisfactory if the device readings are not lower than 1.24. Their difference for different battery jars should not exceed 0.03 units. If the density in the banks corresponds to the norm, you should return the plugs to their place and begin using the battery.
What is density and what does it affect?
An essential element of a lead-acid battery is the electrolyte. This is sulfuric acid diluted with distilled water. The density of water is 1 gram per milliliter (g/ml). For sulfuric acid it is higher than for water, and is 1.84 g/ml. Concentrated acid can dissolve many metals, including lead, so it should be diluted with water. An acid diluted with water is called an electrolyte. It is diluted to a proportion at which it is unable to dissolve the lead, but allows a chemical process called electrolytic dissociation (a type of electrolysis) to occur.
The higher the density, the stronger the electrolysis, but the faster the destruction of lead. The most optimal density for batteries is 1.27 g/ml for temperate climate areas with an average temperature of -20 to +30°C. This is the density of a fully (100%) charged new battery. For northern regions this value is 1.29 g/ml, for southern hot climates - 1.25 g/ml.
Sulfuric acid goes on sale already in the form of an electrolyte with a density of 1.3 g/ml. Taking into account the operating conditions of the battery, it is adjusted to the required parameters.
As noted above, the greater the density of the electrolyte, the stronger the electrolysis and the higher the potential at the battery terminals. The new battery has a density of 1.27 g/ml and a voltage at the terminals of 12.8 V. During the operation of the battery, with regular undercharging, insoluble lead sulfate, a compound of sulfuric acid with lead, forms on its lead plates. This is called plate sulfation. When the battery is charged, not all the acid is released, and the density of the electrolyte decreases. And consequently, the intensity of electrolysis decreases. The voltage at the terminals will be less than 12.8 V. And an attempt to charge the battery to the initial voltage value only leads to boiling of the electrolyte - the active release of hydrogen and oxygen bubbles. This is the process of water decomposition. Loss of water leads to increased density.
Expert opinion
Alexey Bartosh
Specialist in repair and maintenance of electrical equipment and industrial electronics.
Ask a Question
Too high or low density is equally unacceptable and significantly reduces the service life of the battery.
Under vehicle operating conditions with frequent engine starts and short mileage, accelerated sulfation of the plates occurs and a decrease in the density of the electrolyte. When operating vehicles on long-distance flights with long-term engine operation, the battery is recharged and water decomposes into gases, and the density of the electrolyte increases. The voltage at the terminals no longer reflects the state of charge of the battery. And in order to accurately determine the condition of the battery, you need to measure the density of the electrolyte. A hydrometer is used for this.
A hydrometer is a device for measuring the density of liquids and solids, the operating principle of which is based on Archimedes' Law.
Reasons for distortion of the density readings of the solution in the battery
Before checking the density of the electrolyte in the battery, it is important to understand the reasons that led to its sharp decrease. The main reasons for changes in density not related to the operating discharge of the battery are:
- Evaporation of the solution.
- Leakage of electrolyte through seals in traffic jams due to the fact that the car was driven off-road.
- Increased discharge of the battery, leading to the deposition of acid on the plates (sulfation effect).
Let's look at each of these reasons in more detail.
Evaporation and boiling of electrolyte
Intense evaporation of the electrolyte is possible due to long-term operation of the battery located in close proximity to the heated engine. As a result, in very hot weather it can “boil”, which is accompanied by abundant release of gases. Therefore, you should be very attentive to the condition of the battery at elevated air temperatures.
For the same reason, in the summer it is recommended to systematically check the density of the heated electrolyte (at least once a week). If evaporation of the solution is detected, it is enough to add a certain amount of distilled water to the jars. Topping up must be accompanied by mandatory stirring of the resulting solution.
Leakage of liquid solution
If traces of electrolyte splashing out or leaking from the battery cans are detected, the missing amount of solution should be added to them.
But first you will need to remove the spilled mixture from the surface of the battery using a clean rag soaked in ammonia or a solution of soda ash.
The top of the battery housing must be kept clean at all times. If it is heavily contaminated with a mixture of electrolyte and oil splashes, mechanical cleaning will be required. To do this, you can use a brush with stiff bristles.
Sulfation of plates
The formation of a continuous deposit of lead sulfate (sulfation) on the battery plates is the result of improper operation of the battery. The reasons for this effect are as follows:
- The battery was stored in a discharged state for a long time.
- Its operation at low or high temperatures.
- Frequent short trips in winter (poor battery charging).
- Incomplete charging of batteries during routine maintenance.
Important! The most favorable conditions for sulfation are when the battery is discharged to the minimum level (1.18).
The decrease in its performance is explained by the fact that solid crystals deposited on surfaces impair conductivity and reduce the discharge current. The process of plaque formation that has begun can be stopped by using special charging techniques.
Dependence of density on the degree of charge
For the electrolyte of a car battery with a normal density of 1.27 g/cm3, the following dependences on the degree of battery discharge can be given:
| Density g/cm3 | Charge level | Freezing point |
| 1.27 | 100%, | – 60°С; |
| 1.26 | 95%, | – 55°С; |
| 1.25 | 87%, | – 50°С; |
| 1.24 | 80%, | – 46°С; |
| 1.23 | 75%, | – 42°С; |
| 1.22 | 70%, | – 37°С; |
| 1.21 | 63%, | – 32°С; |
| 1.20 | 56%, | – 27°С; |
| 1.19 | 50%, | – 24°С; |
| 1.18 | 44%, | – 18°C; |
| 1.17 | 37%, | – 16°С; |
| 1.16 | 31%, | – 14°С; |
| 1.15 | 25%, | – 13°С; |
| 1.14 | 19%, | – 11°С; |
| 1.13 | 13%, | – 9°C; |
| 1.12 | 6%, | – 8°С; |
The table of electrolyte density shows the dependence of density and freezing temperature. The data presented show that deep discharge of the battery can lead to its freezing even at a temperature of 8 - 16 ° C
Preparing the battery for the winter
In winter, when starting the engine, the load on the battery unit increases, which forces us to prepare it for operation in critical conditions. Preparatory activities are usually carried out in two stages. At the first stage, the battery is thoroughly washed with distilled water, and then filled with new electrolyte and charged to normal. It is important to check the density of the electrolyte in the battery before and after charging it. At the second stage, reliable insulation of the battery is organized within the engine compartment.
Washing and charging
The procedure for flushing the battery during preparation for winter does not differ significantly from the previously described procedures. The same can be said about increasing the density of the electrolyte by passing direct current through it. In this case, the indicated indicator should correspond to the winter norm, that is, have a value close to 1.25-1.24. This process differs from summer maintenance in this way.
When and what to top up the battery with?
The need to add working fluid to the battery occurs infrequently, but it is sometimes necessary. What, how much and in what cases should I add? There are two such cases: low electrolyte level and abnormal acidity of the working fluid.
We recommend: Sznajder – car batteries
Low level in sections
This situation occurs often because during battery operation the water evaporates or, as they say, boils away. In this case, the level of solution in the sections decreases, and the edges of the plates turn out to be dry. This can be determined visually by simply unscrewing the plugs from the sections and looking into the filler necks. The normal liquid level in the section should be approximately 1 cm above the cut-off level of the plates. Some batteries even have a special mark stamped on the case. If the level is low, then the situation, although serious, can be easily eliminated. For this operation you will need:
- a medical syringe without a needle or a car hydrometer;
- distilled water;
- protective equipment (glasses and rubber gloves).
Distilled water is drawn into a syringe and poured into the appropriate sections to the required level. After adding liquid to the battery, it is placed on charge. In this regard, an autohydrometer is much preferable, since by adding water, you can immediately control the density of the solution.
Be careful not to work with acid unless your eyes are protected.
Why is a full battery charge necessary?
- The more often the lead plates in the battery become rarefied, the faster the sulfation process occurs.
- The quality of the battery is directly proportional to the density of the electrolyte.
Notes:
- Battery maintenance should be carried out every season. Especially during the autumn-winter period.
- The battery becomes unusable for various reasons. Often with long engine starts using the starter. In winter, the starter consumes increased current and can lead to warping of the plates.
- The charge of the battery is directly proportional to the density of the electrolyte and the degree of charge.