Many motorists have probably heard, but some have never seen, how they add acetone to fuel and get incredible results in increased power, fuel economy and other benefits.
There is another category of motorists who have encountered the opposite effect, and in fact had an extremely negative experience of mixing gasoline with acetone. And here it is important to figure out who is right and who is wrong, what acetone actually does and whether it makes sense to add it to the gas tank at all.
It is necessary to clearly understand what advantages or disadvantages the car and specifically the engine will receive if regular gasoline is diluted with this solvent. Some people don’t even understand where this idea came from and why acetone is sometimes so actively promoted as an incredibly effective additive.
How it all started
To begin with, it should be noted that acetone is primarily used as a solvent. Although sometimes it serves as the main reagent necessary to obtain substances of a more valuable category through chemical reactions.
The world first learned about acetone, which was then called pyroacetic ether, in 1732. It was in this year that acetic acid was distilled using the dry method, obtaining a new substance. But only 100 years later, the currently used chemical formula of acetone was approved, as was the updated name itself.
In fact, acetone can be described as a highly mobile, colorless liquid that smells like mint. Thanks to its properties, the substance mixes perfectly with fuel, alcohols, water, and organic solvents. Acetone can also mix immiscible substances, creating a homogeneous solution.
One of the most valuable properties of acetone is its ability to create autogas. Without taking into account all other characteristics, it is safe to say that it can dissolve excellently.
If we talk about the idea of adding acetone to the gas tank, it did not arise by chance. The thing is that the substance has an incredibly high octane number. From here, someone started a logical chain, according to which in order to increase the octane number of the fuel, it is enough to simply add the corresponding component to it. And here acetone came in very handy.
Information that adding acetone to a car's gasoline tank is very useful and effective appeared in 2005. A material was published on a foreign resource that experts managed to discover a new type of alternative fuel for cars. Allegedly, many experiments were carried out in which gasoline was mixed with a solvent in various proportions. A 4-stroke engine with 1 cylinder and a spark ignition system was used as a test subject.
The following were the results of a study in which the mixture was more effective than regular pure gasoline. Experts noted an increase in exhaust gas temperature, an increase in cylinder pressure, as well as an increase in torque and overall engine efficiency. But the increase was no more than 2.3% of the nominal value. The same torque increased by only 0.45%.
When I tried to add more for the experiment, nothing surprising happened. The substance simply evaporated, and during combustion large volumes of hazardous substances were formed.
But the problem is that the public learned about the results of the first studies only several years after the experiments were carried out. The results were summed up in general terms and some numbers that were not supported by more detailed explanations. At the same time, everything looked so convincing that motorists hastily began to buy acetone and pour it into the gas tanks of their cars.
There were also episodes of foreign and domestic television programs where similar experiments were staged. As a result, it was shown that when mixing components, fuel consumption increases, and when a large amount of solvent is added, it simply evaporates.
Review and comparison of White Spirit and Solvent 646
White spirit, which is widely used in repair work, has similar qualities. The composition of this type of solvent is a mixture of aliphatic and aromatic hydrocarbons obtained through processing and subsequent hydrotreating of oil. Liquid formula 646, in addition to alcohols and aliphatic components, contains acetone. The substances have almost the same density. However, they differ in other technical characteristics, the main ones of which are reflected in the table.
| Type of mixture | White Spirit | Solvent 646 |
| Boiling temperature | 160 degrees | 59 degrees |
| Flash point | 33 degrees | 7 degrees |
| Density | 0.795 g/cm 3 | 0.87 g/cm3 |
| Safety | non-toxic | toxic |
Is it worth adding
First, you need to understand why motorists add acetone to gasoline. They rely on recommendations from other people, some articles of dubious origin, publications in specialized magazines, etc.
Everyone decides for themselves why people pour technical acetone into their gas tank. This is mainly due to the desire to increase the efficiency of the internal combustion engine, clean it of accumulated contaminants, and also raise the engine parameters at least to the original ones when the car was just released from the assembly line.
The desire to save on fuel and improve the performance and condition of the engine sometimes forces motorists to take risks, experiments, and rely on research results that are not supported by real evidence or are not officially approved.
One of these risks is mixing fuel with technical acetone. But in practice, it is difficult to predict exactly how the motor will behave in the event of an experiment, and how destructive or positive the consequences will be. This is influenced by a number of characteristics. Namely:
- quality of source fuel;
- car condition;
- driving style;
- engine's type;
- design features of the motor, etc.
In fact, acetone can be considered an additive or additive. But if you listen to operation, repair and maintenance specialists, most of them are against the use of additives so popular in our time. The only exceptions include those drugs that the car manufacturer itself recommends filling into the tank. However, acetone is definitely not included in this list.
It is logical to assume that if there is no recommendation or permission to mix fuel with acetone in the instruction manual, then you should not do it. But our motorists rarely listen to the manufacturer, and do what they themselves think is right. Therefore, there are a number of examples when solvent was poured into a gas tank, and car owners faced various consequences.
The question is not even whether it is possible to add acetone to a car’s gas tank and drive a car with such a mixture. Here it is rather necessary to raise the question of the appropriateness of such a decision. And to do this, you should consider 2 popular myths directly related to the addition of solvent.
Myth one. Improving combustion efficiency
There are a number of people who claim that fuel combustion efficiency increases when a solvent is added to it. At the same time, they name figures from 10 to 40% in comparison with nominal values. As arguments, the statement is given that acetone tends to reduce the surface tension of fuel molecules.
If we take a conventional engine as a basis, then fuel combustion occurs almost completely. This is especially true for serviceable engines that do not have any problems with the fuel system or combustion chambers. A logical conclusion arises here. In order for combustion efficiency to increase by 10%, not to mention 40%, it will be necessary to burn the fuel 10% better. It is physically impossible to do this, and acetone is not capable of dramatically increasing the energy gain, given the small proportions in which the solvent is added.
Myth two. The engine is cleaned and combustion products are dissolved
Yes, everyone already knows that acetone is a solvent. But here it is also important to focus on its properties. Solvents are divided into polar and non-polar. In the case of the substance in question, we are talking specifically about a polar type of solvent. But gasoline is non-polar. They cannot mix with each other.
A simple ketone has become a superstar
At one time, the program “MythBusters” began to gain momentum, where the two main presenters either confirm various exciting and interesting statements or refute them. Their curiosity did not leave our main character of the article aside. “MythBusters” added acetone to gasoline in the 58th episode of their television epic, where they experimentally established that with such exposure, consumption still increases. For TV viewers who are not impressed by foreign programs, you can find domestic analogues. Although “Mythbusters” added acetone to gasoline first, in “Main Road”, in the issue dated 2009.06.12, they went further - they poured pure acetone, it evaporated instantly. However, stories about the power of this substance excited minds, which led to the emergence of myths and legends.
How much can you add?
Many who mix acetone and gasoline, or want to test the performance of the engine when mixing fuel with solvent, are not sure what proportions should be used.
In fact, there are no clear official instructions or recommendations that say how much acetone can or should be added to the gasoline in the tank. No one officially recognizes this solvent as a useful and effective additive. All information about the effect of a substance on fuel is exclusively unofficial and largely speculative.
As a result, motorists begin to experiment and pour different amounts at their discretion. One pours several liters at once into a half-empty tank, others pour 50 ml into a full tank. Today we will fill a liter, and tomorrow we will try with 2 liters. There are many situations, and in each of them there are conflicting results.
To clean the engine, when acetone is added to gasoline, the proportions according to the recommendations are 1 to 10. Supposedly, this is how it is possible to obtain high-quality cleaning without harmful consequences for the engine.
Depending on why technical acetone is added to gasoline, the proportions and ratios of substances vary. If you do not want to take risks, but curiosity takes over, you can add no more than 10% of the total volume available in the tank.
But a slightly different norm is considered more acceptable. It provides for the use of no more than 300 ml of solvent per full 50-liter tank. Moreover, it is important to use exclusively pure, high-quality technical acetone.
What to replace
If the quality of gasoline is not satisfactory, it is recommended not to use folk remedies, but to buy special additives. There are 4 classes of such substances:
- To remove water from the tank. Allows you to effectively remove moisture accumulations from the fuel tank.
- Cleaners. Designed to clean the fuel system of injection and diesel engines.
- Octane number correctors. Recommended for use when using low-quality fuel. The additive will help increase dynamic performance and reduce fuel consumption.
- Universal additives can solve all of the above problems to one degree or another.
Remember, a good specialist will never advise under any circumstances to add acetone or any other solvent to the gas tank. The consequences of such actions are dire.
When an experiment is beneficial
Nowhere in official sources, authoritative publications, or statements from automobile additive manufacturers will you find confirmed data that acetone is useful and effective when added to fuel.
But there are still a huge number of reviews online that say that by adding acetone to gasoline, they were able to significantly increase engine performance, reduce fuel consumption, clean the engine of contaminants, and other positive opinions. But for every such positive review there is a completely opposite opinion and statement.
Whether to believe in the miraculous properties of the solvent or not is up to each individual. It's your right to experiment on your own machine. Only you will also be responsible for their consequences.
Environmental friendliness
Modern engines are manufactured taking into account global and local environmental standards in order to reduce the level of bad emissions. And if the mixture of gasoline and oil is quite predictable and studied, then with the addition of acetone the exhaust becomes more toxic. Considering that cars have special sensors that monitor the composition of exhaust gases, if acetone vapor is detected, they can turn on a reduced power mode. The consequence is that the car will become more harmful to the environment and will also drive worse.
Advantages
Although acetone looks like an extremely dubious solution for performing the tasks assigned to it, it is still necessary to talk about some of the positive properties and capabilities of this solvent.
- Acetone, used as an additive in gasoline, has a high concentration of oxygen. Such conditional additives stimulate better mixing of the air-fuel mixture before ignition, which is also due to the excellent volatility of the solvent. This will cause the mixture to burn faster. In theory, this gives an increase in fuel economy and increased torque.
- Acetone is even used in fuel production. Only here the concentrations are minimal, since manufacturers make detailed calculations and check the properties of their fuel under certain conditions.
- There are claims that in winter, adding solvent makes the engine start easier. But here one can cast doubt on such a positive point, since much depends on the specific temperature. The solvent has a freezing point of -20 degrees Celsius. That is, under such conditions, you are more likely to get harm than benefit.
- Resistance to detonation. Another advantage, which, like all the others, has not been confirmed, but also not officially refuted. Self-ignition of gasoline, to which no separate additives have been added, occurs at 257 degrees Celsius. For acetone, this figure reaches 465 degrees. Thus, mixing gives improved detonation resistance of the mixture.
- Removing water. Condensation in the tank forms gradually, even with a fully functional fuel system. At the same time, acetone is one of the cheapest additives that can rid the tank of excess moisture.
It is impossible to say that these statements are 100% correct. So far, all information is based on assumptions, theory and practice only by ordinary motorists who took a conscious risk and mixed 2 components in the tank of their car.
But before you pour acetone into the gas tank, you need to talk not only about the advantages, but also about the existing disadvantages.
What is detonation?
Detonation is the premature ignition of the air-fuel mixture. When the piston moves upward, a spark is supplied even before top dead center.
And the fuel should gradually ignite, thereby pushing the piston when it begins to move down. That is, the temperature of the gases rises, and they push the piston.
What happens at the moment of detonation?
The fuel ignites chaotically and very quickly. It turns out that the gases no longer push it so effectively at the moment of lowering, so power is lost.
Fuel consumption increases and the piston itself suffers greatly, since the fuel ignites very quickly.
Literally in a split second everything burns out, but it should burn gradually and push the piston smoothly.
Many people say that you can fill in absolutely any gasoline in modern cars, because there is a knock sensor there, which supposedly regulates all these combustion processes.
But in reality this is a little different.
The knock sensor only gives a signal to the control unit after premature ignition of the fuel, that is, when engine vibration occurs.
All this is monitored in the form of a graph, and the control unit is already beginning to regulate the ignition angle, or rather, it decreases it, makes it later, and accordingly the air-fuel mixture will ignite later.
Even if low-octane gasoline ignites sharply, it is not so bad, because the spark will be supplied when the piston goes down. But this takes time.
The sensor cannot give signals in advance and it turns out that detonation will still occur for the first fraction of a second.
This is how it affects the engine. So think about it.
In general, I looked for this gasoline. As you heard, there is a slight detonation, not constantly, but it appears periodically.
Possible consequences
Opinions regarding the use of solvent as a fuel additive are divided into positive and negative. Moreover, according to some sensations, there are much more adherents of the latter.
If you study the reviews in more detail, it becomes clear that an impressive number of motorists who took risks speak negatively about the effect of the substance on engine performance. In their opinion, acetone and gasoline provoke only negative consequences.
Moreover, the situations are very diverse. Some write that adding even a small amount of acetone to gasoline led to the car jerking, the power unit choking and severe contamination of the injectors after opening the engine. For others, the car drove about 100 kilometers, everything was fine, but at one point the engine simply stalled and stubbornly refused to start.
Strong solvent
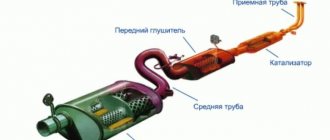
Acetone is a fairly strong solvent that can (surely) dissolve deposits in engine cylinders, but can also damage non-metallic fuel lines and seals.
It is also unknown exactly how acetone can affect motor oil, and contact between them cannot be avoided. Although in theory oil should not enter the combustion chambers, it still penetrates there in small quantities, and on some machines in large quantities. The contact between oil and acetone can be especially strong when starting a cold engine, when there are difficulties with the evaporation of gasoline that is supplied to the cylinders. Taking into account the fact that evaporated gasoline (or diesel fuel) can condense and come into contact with the cold walls of the cylinders, this can deteriorate the quality of lubrication of the upper zone of operation of the piston rings. In theory, this will affect durability, but exactly how it will happen in practice, no one can say for sure.
Who is interested in adding acetone to fuel?
You won't be surprised if it's the manufacturers themselves. But there are other people who also support the idea of mixing acetone and gasoline. And these are conservationists.
A number of enterprises and industrial facilities actively use acetone as an organic solvent. It is capable of dissolving synthetic-based fabrics, plastic, etc. It is also a key component in the production of paints and varnishes.
Actively using a solvent, it ends up among numerous wastes. At the same time, a wide variety of disposal methods are used, if at all they are used by some individual organizations. The simplest and cheapest is burial in the ground.
But with this supposed disposal, acetone penetrates into the soil and then gets into the water, which leads to their contamination. Animals and people drink all this, plants grow on it.
There is another method that involves burning. But then the acetone evaporates and is released into the atmosphere. It is also extremely bad for the environment, which has already suffered at the hands of man. A high level of acetone concentration in the atmosphere and the air you breathe provokes problems with the nervous and reproductive systems, causes kidney disease, destroys the liver and more.
Environmentalists call catalytic incineration the most correct disposal method. But this requires special installations, which are expensive, and few people want to do this because of the financial component. But as a result of such combustion, acetone simply breaks down into safe water and carbon dioxide.
To find a cheap way out of the situation, environmentalists began to support the idea of adding solvent to the gas tank. After all, this is how catalytic combustion can be achieved.
The manufacturers themselves are also not against such experiments, since they produce a solvent that is actively sold. And the average cost of 500 ml of the substance is now 70-80 rubles.
As a result, it turns out to solve several problems simultaneously:
- make a profit;
- increase demand;
- recycle.
But in fact, environmentalists and acetone producers sacrifice motorists, their cars, and the money they spend first on purchasing the solvent and then on eliminating the consequences of adding it.
How to wash dirty injectors with acetone through a tank: question technique
Despite the wide range of branded additives, experienced drivers are in no hurry to abandon folk remedies. This is understandable. The cost of a liter of acetone does not exceed 150 rubles. The savings are obvious, but let's talk about the effect.
The drug itself does not have cleaning properties. Let's say he washes fuel oil from his hands no better than a household Fairy. And with the latter, even washing the engine
not effective. But it has not yet been added to gasoline. Once you mix them, it immediately becomes clear that this mixture has excellent cleaning properties.
Cleaning the engine from carbon deposits
It has been proven that thanks to this product, dirt and carbon deposits that appear on the injectors and spark plugs of the car are cleaned. It makes idle smoother and increases traction at low speeds.
You should not pour acetone into the tanks of new cars. Such experiments are only appropriate for owners of cars whose mileage is over 100,000 km.
Proportions for filling: for 50 liters of gasoline, about 250 ml of pure solvent (it is important that it does not contain any additives).
Who started it first?
The origins of the popularity of acetone take us back to 2005, when an article appeared on a foreign website about a newly discovered new alternative fuel. It said that several experiments were carried out with the addition of acetone to gasoline, the proportions were 3/97, 7/93, 10/90 of the total volume as a percentage (acetone/gasoline).
A single-cylinder, four-stroke, spark-ignition engine (internal combustion engine) was used for the experiment, and the results showed that the blended fuel had several advantages over pure gasoline, namely in exhaust gas temperature, cylinder pressure, torque and efficiency by approximately 0.8% , 2.3%, 0.45% and 0.9% (at a ratio of 3/97). When the acetone content was increased to 10%, the performance improved by 5%, 5.2%, 2.1% and 3.2%. Then we thought and asked the question: “What will acetone give in gasoline if there is more of it?” Here, in principle, no one was surprised; the acetone quickly evaporated, and the products of its combustion were a whole range of harmful substances.
By the way, no one provided the results of the combustion characteristics of acetone, which were obtained during the first experiment; they were made available for review much later, which made it possible to continue further experiments. Inquisitive minds were unstoppable. The Internet is full of good reviews, assurances, vows, various graphs and laboratory studies. All the evidence seemed so convincing and plausible that only the lazy did not try to pour at least a drop of acetone into the gas tank.
What can I say! They are convincing and believable even now. And many do not understand who to believe. The time has come to lift the veil of secrecy.
Removing excess condensate
Another positive effect will have a solvent mixed with water. This is especially true when condensation collects in the tank and is not completely removed. In this case, the solvent will help completely remove the liquid.
Of course, you can use more expensive means for this purpose, but it cannot be said that they are much more effective.
Knock resistance
An additional advantage of a fuel mixture containing acetone is its resistance to detonation. It is known that gasoline spontaneously ignites at a temperature of 257 degrees (without additives), and the self-ignition limit of acetone is 465 degrees. Consequently, its presence in the mixture increases the knock resistance of the fuel, and this reduces the likelihood that it will ignite before the spark plug creates a spark. According to reviews, the consequence of acetone in gasoline is the disappearance of the phenomenon of engine detonation (when the fuel ignites prematurely).
Common Myths
Myth No. 1 “Acetone is an excellent helper against deposits and carbon deposits in the engine”
Let's find out if this is true. It's worth starting with the fact that acetone is polar, and gasoline is non-polar. Substances of different polarities do not mix. However, acetone and standard interact well with gasoline, but despite this, they are still of different polarities. Inside the car’s engine, there are constant flows of oil and gasoline - they do not form serious deposits.
Even if something has “grown”, acetone is not able to cope with it. The reason lies in the polarity of the chemical compound. Let's say that deposits have appeared in the internal combustion engine. In this case, in order to remove them, you need to disassemble the engine. So, the myth that acetone can clean the engine from the inside is dispelled. The only thing that can be achieved by adding acetone to gasoline is the elimination of minor contaminants on the injectors (this is only relevant if you are the owner vehicle manufactured before 1995).
You should also not ignore the fact that the fuel system has various hoses, seals, etc. Therefore, regular use of acetone can lead to leakage and damage to the nozzles, which are able to withstand non-polar solvents, but they are polar ones.
Myth No. 2 “Adding acetone can increase the octane number, which will contribute to less gasoline consumption”
You've probably come across articles on the Internet, reviews on forums, that the octane number of a solvent reaches 100, or even exceeds this value. But such information is completely incorrect. The fact is that acetone simply does not have an octane number - this is a completely different chemistry, the formula of which is aimed at dissolving paintwork materials, and not for ignition in the engine.
Let's turn to arithmetic and calculate how much 1 liter of gasoline + solvent will cost. So, 1 liter of 92 gasoline this year costs 41.98 rubles, and half a liter of solvent costs 86 rubles. With a tank volume of 50 liters, 5 liters of solvent will be required to increase the octane number. Thus, 45 liters. gasoline will cost 1889 rubles, and 5 liters of acetone - 860 rubles. As a result, we will have to pay for the mixture - 2747 rubles. Based on this, 1 liter of such a mixture will cost 54.94 rubles. The question arises, how much will it be possible to increase the octane number?
Also, another question immediately arises: is the mixture costing 54.94 rubles 95, 98, or 100 gasoline? To sum up, it is worth saying that the octane number of 92 gasoline will not be raised. Why? The fact is, as mentioned above, acetone simply does not have it, which means that from 92 gasoline it is impossible to get 98, 100. In connection with this, using a solvent will not be able to raise the octane number of gasoline and have a positive effect on it characteristics. Therefore, mixing gasoline with acetone will not make your car drive any better!
Myth No. 3 “Acetone + gasoline is the best solution for increasing car power”
You've probably come across reviews online more than once, opinions that mixing gasoline with acetone will add 5-10 horsepower. But is this really so? To increase the power of the car you will need to install a turbo system or replace the engine with a more modern one. As for acetone, its main purpose is to dissolve paints and varnishes, but not to increase engine power.
But there is an opinion that if you buy acetone solvent and mix it with gasoline, you will be able to improve the quality of the fuel, which will have a positive effect on engine power. This opinion is wrong. During the experiment, it was revealed that by adding acetone to gasoline, the octane number did not increase, and engine power did not improve. If this method is not effective, why are there so many rave reviews online? They do this in order to increase the volume of sales of acetone for other purposes.
Of course, if those who simply thoughtlessly copy material from the network and distribute it, they themselves have never tried to carry out such an experiment to verify this ineffectiveness. We () have been producing solvents, including acetone, for many years. Therefore, he speaks with knowledge of the matter that a solvent, when mixed with fuel, is not able to increase the power of a car and will in no way improve its driving characteristics.
Myth No. 4 “Acetone removes detonation in internal combustion engines”
Initially, it is worth finding out what detonation in an internal combustion engine is? This concept means a violation of the combustion process of the fuel-air mixture. This phenomenon takes on an explosive shock character. You can encounter it if the car was filled with poor quality fuel. For example, instead of the required 95-grade gasoline, they filled in with 92-grade gasoline, which led to poor engine performance.
And many believe that pouring acetone into the tank will improve the quality of poor fuel. However, this is not true because the solvent has no octane rating, which means it cannot improve the fuel. In turn, this indicates that detonation in the internal combustion engine will not disappear. What to do? If there is detonation, the best solution would be to use gasoline of a higher class. This myth has also been dispelled. Acetone is not able to remove detonation in the engine, since it does not have an octane number, which means that it cannot in any way affect the quality of the fuel.